Understanding EXHAUST EMISSIONS
Five Gas Exhaust Analysis Theory
Use of a four or five Gas Exhaust Analyser can be helpful in troubleshooting both emissions and driveability concerns. Presently, shop grade analysers are capable of measuring from as few as two exhaust gasses, HC and CO, to as many as five. The five gasses measured (for petrol emissions) by the latest technology exhaust analysers are: HC, CO, CO2, O2 and NOx. All five of these gasses, especially O2 and CO2, are excellent troubleshooting tools. Use of an exhaust gas analyser will allow you to narrow down the potential cause of driveability and emissions concerns, focus your troubleshooting tests in the area(s) most likely to be causing the concern, and save diagnostic time. In addition to helping you focus your troubleshooting, an exhaust gas analyzer also gives you the ability to measure the effectiveness of repairs by comparing before and after exhaust readings. In troubleshooting, always remember the combustion chemistry equation: Fuel (hydrogen, carbon, sulphur) + Air (nitrogen, oxygen) = Carbon dioxide + water vapour + oxygen + carbon monoxide + hydrocarbon + oxides of nitrogen + sulphur oxides.
When we do exhaust analysis, we are being a detectives. We look at what came out of the exhaust and figure out what could have happened before to create those emissions. What happened in the combustion chamber, or before the combustion chamber, to create these results?
We can use clues and patterns of exhaust readings to figure out if we have a problem in one of the following areas:
- Air/Fuel Ratio
- Combustion
- Ignition
- Emission Control Devices
Then we know where to start our diagnosis with visual and functional tests. If we know that the combustion in our engine is OK and efficient, there isn't much left to worry about. But how do we know good combustion from a bad one? Let's find out...
Complete (Good) Combustion:
Let's start by reviewing good combustion. The idea is to properly burn up all the petrol and not have any "leftovers". Into the combustion chamber we put petrol, symbolized by 'HC' for hydrocarbons. These are combinations of hydrogen and carbon atoms. We also add lots of air, which contains oxygen, symbolized by 'O2'. Normal air is about 20.7% oxygen, and if your workshop gas analyser doesn't show about this when reading the air inside your shop, you could have a bad oxygen sensor in your gas analyser( those are chemical sensors and have expected life of about one year), or a serious problem with the air in your shop, or the planet has a problem... Back to combustion. The air we add to the combustion chamber is mainly nitrogen, about 78%. (No, that's not nitrous, but related.) This doesn't burn, it just goes along for the ride and expands with the heat, helping to push down the piston.
Coming out of the combustion chamber we have carbon dioxide, water and nitrogen. The carbon dioxide is symbolized CO2. (One carbon atom combined with two oxygen atoms) It's good, in that plants like it and it doesn't hurt us, but is blamed too much for global warming. The water is symbolized by H2O, two hydrogen atoms combined with one oxygen atom. Did you realize that for every gallon of petrol we burn, the tailpipe puts out about a gallon of water? And then good combustion also puts out all the nitrogen that came in.
Good combustion is simply put this way: HC + O2 + N2 = H2O + CO2 + N2. Ideally, what we want is to convert all the Fuel and Air that enters the engine in to Water and Nitrogen!
We want an ideal mixture of 14.7 pounds of air to 1 pound of gasoline for the cleanest burning. (14.7:1 Stoichiometric ratio, is the air to fuel ratio at which there is just enough air to burn certain amount of fuel completely.)
There are a few other exhaust components which impact driveability and/or emissions diagnosis, that are not measured by workshop Gas analyzers. They are:
-
Water vapour (H2O)
-
Sulphur Dioxide (SO2)
-
Hydrogen (HO)
-
Particulate carbon soot (C)
Sulphur dioxide (SO2) is sometimes created during the combustion process from the small amount of sulphur present in gasoline. During certain conditions the catalyst oxidizes sulphur dioxide to make SO3, which then reacts with water to make H2SO4 or sulphuric acid. Finally, when sulphur and hydrogen react, it forms hydrogen sulphide gas. This process creates the rotten egg odour you sometimes smell when following vehicles on the highway. Particulate carbon soot is the visible black "smoke you see from the tailpipe of a vehicle that's running very rich.
Incomplete ( BAD) Combustion:
Now for Bad Combustion. This is where the wrong things happen, and the by-products of combustion produce gases which contribute to air pollution or other problems. The Complete Combustion is impossible to obtain even with the best tuned engines! So in practice we are left with Incomplete Combustion and an obsolete engine design. ( When was it invented,.... anyone? too young to remember...) One example of Incomplete Combustion is raw gasoline (HC) which goes in, then comes out, and isn't burnt up in the process. Another example is Carbon Monoxide (CO). It doesn't create smog, but it's deadly, so you don't want it around. A third example is Oxides of Nitrogen (NOx). It helps create out brown smog. These are all a problem and we are soon going to talk about them in more detail. But first, look at what it takes to create photochemical smog:
HC + NOx + Still air + Sunlight = Smog. Get the idea? The HC and NOx are what it takes to create smog, so if we prevent them from coming out of the tailpipe, we can cut down on the smog.
In any diagnosis of emission or driveability related concern, ask yourself the following questions:
-
What is the symptom?
-
What are the "baseline" exhaust readings? At idle, 2500 rpm, acceleration, deceleration, light load cruise, etc.
-
Which sub-system(s) or component(s) could cause the combination of exhaust gas readings measured?
The following major factors contribute to the overall increase in exhaust emissions levels and degraded vehicle driveability:
-
Lack of scheduled maintenance:
-
Sub-system failures
-
Combination of multiple marginal sub-systems
-
-
Tampering with the engine emissions system and sub-systems ( control unit, sensors, actuators etc. etc.)
-
Removal of emissions sub-system equipment
-
Modification of engine/emissions sub-systems
-
Use of leaded fuels or incompatible additives in closed loop control systems
-
A word to all engine tune-up boys out there. When tuning a ordinary modern engine always keep in mind that the factory that produce it already spend millions to get it right! Your best bet is to bring it back to factory specifications, or waste your time and effort trying to "over tune" it! Believe me, it's a long and lonely road with few and far in between real rewards.
Gas Analyser Measurements:
We need to know what the Gas Analyser measures.
These are the gases that the 4 or 5-gas Gas analyser sees in a petrol engine:
- HC = Hydrocarbons, concentration of the exhaust in parts per million (ppm). = Unburned Petrol, represents the amount of unburned fuel due to incomplete combustion exiting through the exhaust. This is a necessary evil. We don't want it so try to keep it as low as possible. An approximate relationship between the percentage of wasted fuel through incomplete combustion and the ppm of HC is about 1/200 ( 1.0% partially burned fuel produces 200 ppm HC, 10%=2000 ppm HC, 0.1%=20 ppm HC )
- CO = Carbon Oxide, concentration of the exhaust in percent of the total sample. = Partially Burned Petrol, This is the petrol that has combusted, but not completely. This gas is formed in the cylinders when there is incomplete combustion and an excess of fuel. Therefore excessive CO contents are always a sign of an overly rich mixture preparation. ( The CO should have become CO2 but did not have the time or enough O2 to became real CO2 so it is exhausted as CO instead.) CO is HIGHLY POISONOUS ODORLESS GAS! Always work in well ventilated areas!
- CO2 = Carbon Dioxide, concentration of the exhaust in percent of the total sample. = Completely Burned Petrol, represents how well the air/fuel mixture is burned in the engine ( efficiency ). This gas gives a direct indication of combustion efficiency. It is generally 1-2% higher at 2500 RPM than at idle. This is due to improved gas flow resulting in better combustion efficiency. Maximum is around 16%. At night the trees convert CO2 in to Oxygen. Preserve them!
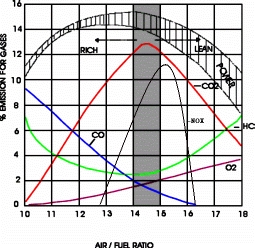
- O2 = Oxygen, concentration of the exhaust in percent of the total sample. Free O2 occurs in the exhaust when there is an excess of air in the mixture. The O2 content increases sharply as soon as Lambda rises above 1. Taken with the CO2 maximum, the oxygen content is a clear indicator of the transition from rich to lean mixture range, or leaks in the manifold or exhaust systems or combustion failures. With rich mixture most of the oxygen is burned during combustion. Whit very lean mixture more O2 escapes "un-combusted" so the level rises.
- NOx = Oxides of Nitrogen (This is only seen by a 5-gas analyser) Only seen with dynamometer or engine under load. NOx emissions rise and fall in a reverse pattern to HC emissions. As the mixture becomes leaner more of the HC's are burnt, but at high temperatures and pressures (under load) in the combustion chamber there will be excess O2 molecules which combine with the nitrogen to create NOx. NOx increases in proportion to the ignition timing advance, irrespective of variations in A/F ratio. This gas is related to the exhaust gas detoxification systems ( in conjunction with Co and HC) , exhaust gas recirculation systems. Those systems bring some of the inert (processed) exhaust gas back in to the engine to be burned again. This time around this gas has no O2 extra molecules and prevents high combustion temperatures and further increase in NOx formation. NOx is Very Dangerous Lethal Gas and air pollutant!
- A/F ratio or Lambda = Calculated Air/Fuel Ratio or Lambda value based on the HC, CO, CO2 and O2 concentrations. Remember the ideal (Stoichiometric) A/F is 14.7 liters air to 1 liter fuel or 14.7/1. The ideal Lambda value is 1(one) below that the A/F mixture is rich and above - lean. For example, lambda=0.8 corresponds to an air/fuel ratio of (0.8x14.7):1=11.76:1 ( e.g. lambda 0.8 = A/F ratio of 11.76/1 or very rich air fuel mixture )
General Rules of Emission Analysis
-
If CO goes up, O2 goes down, and conversely if O2 goes up, CO goes down. Remember, CO readings are an indicator of a rich running engine and O2 readings are an indicator of a lean running engine.
-
If HC increases as a result of a lean misfire, O2 will also increase
-
CO2 will decrease in any of the above cases because of an air/fuel imbalance or misfire
-
An increase in CO does not necessarily mean there will be an increase in HC. Additional HC will only be created at the point where rich misfire begins (3% to 4% CO)
-
High HC, low CO, and high O2 at same time indicates a misfire due to lean or EGR diluted mixture
-
High HC, high CO, and high O2 at same time indicates a misfire due to excessively rich mixture.
-
High HC, Normal to marginally low CO, high O2, indicates a misfire due to a mechanical engine problem or ignition misfire
-
Normal to marginally high HC, Normal to marginally low CO, and high O2 indicates a misfire due to false air or marginally lean mixture
Evaporative Emissions
Up to now, we've only discussed the creation and causes of tailpipe or exhaust emission output. However, it should be noted that hydrocarbon (HC) emissions come from the tailpipe, as well as other evaporative sources, like the crankcase, fuel tank and evaporative emissions recovery system. In fact, studies indicate that as much as 20% of all HC emissions from automobiles comes from the fuel tank and carburettor (on carburetted vehicle, of course). Because hydrocarbon emissions are Volatile Organic Compounds (VOCs) which contribute to smog production, it is just as important that evaporative emission controls are in as good a working order as combustion emission controls. Fuel injected vehicles use an evaporative emissions system to store fuel vapours from the fuel tank and burn them in the engine when it is running. When this system is in good operating order, fuel vapour cannot escape from the vehicle unless the fuel cap is removed.
And finally remember: In nature nothing is lost or gained, only converted! Same rule applies to emission analysis.
Automotive Equipment - in tune with the Future